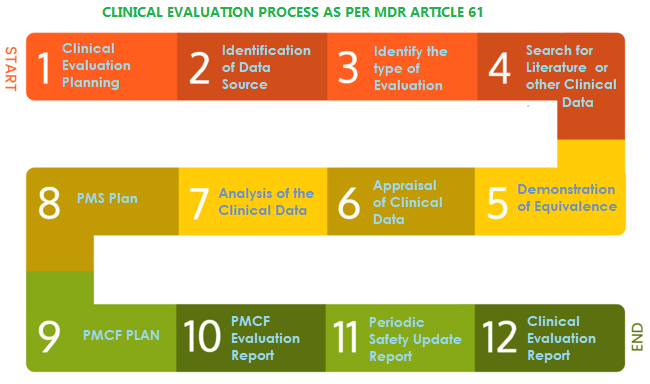
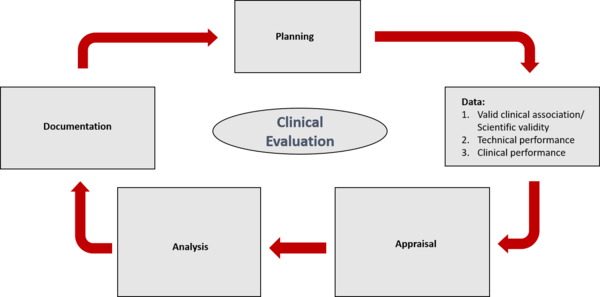
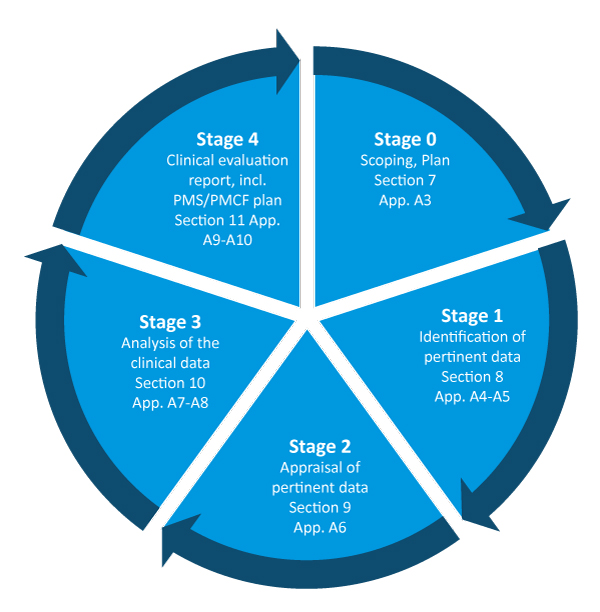
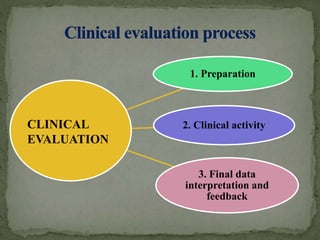
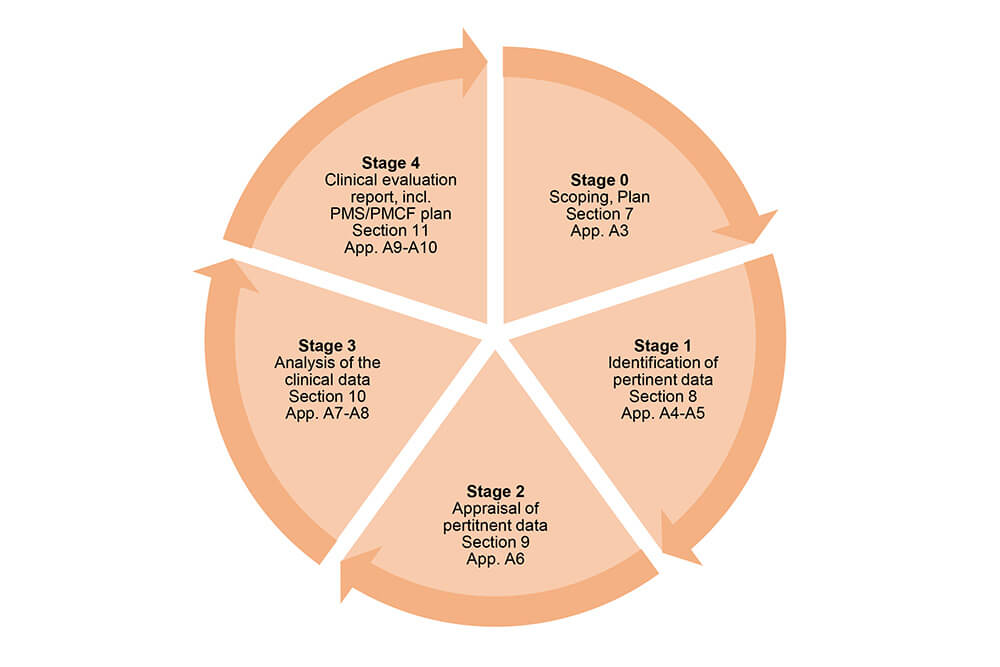
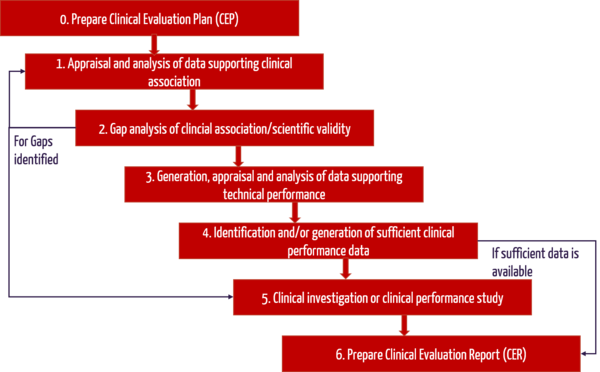
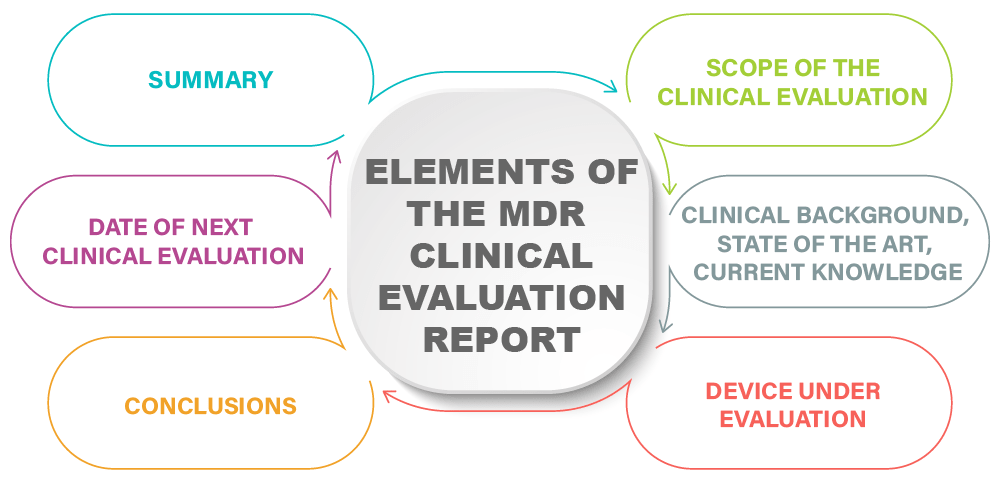
Clinical evaluation: is your Clinical Evaluation Report (CER) compliant with MEDDEV 2.7/1 rev. 4? - Thema Med

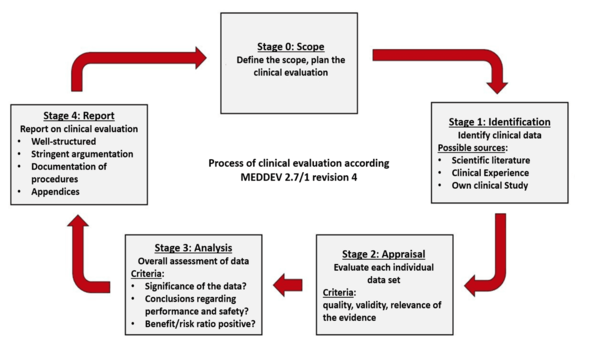
Medical Device Regulation 2017/745 Clinical Evaluation Report: Generalities · MDlaw – Information platform on European medical device regulations