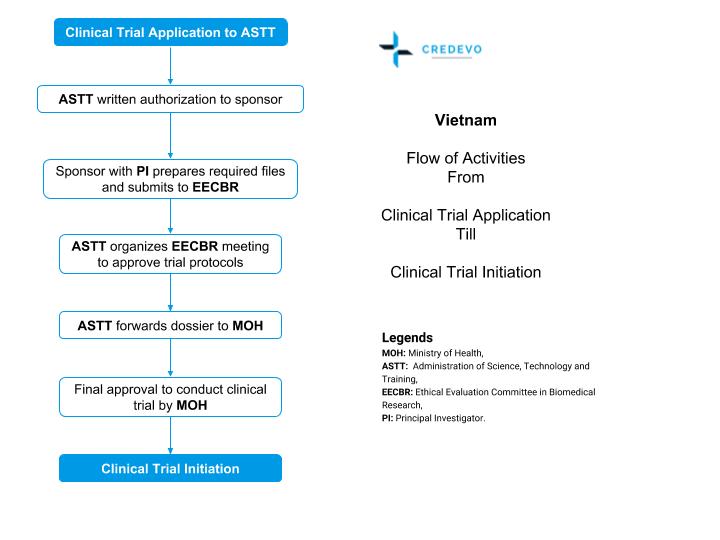
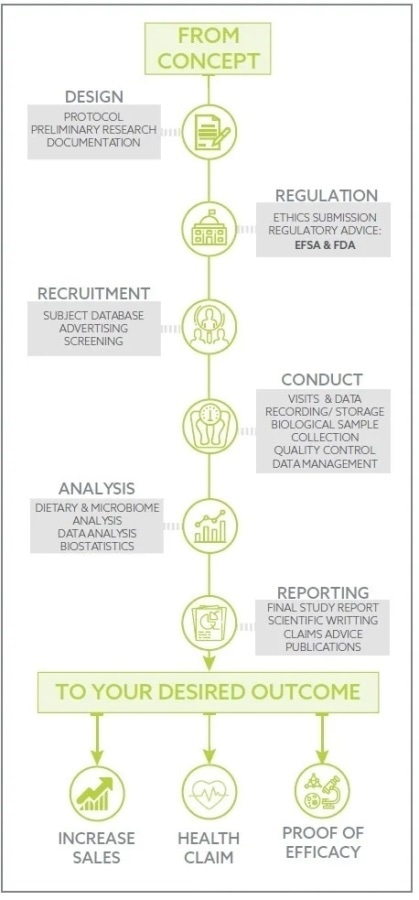
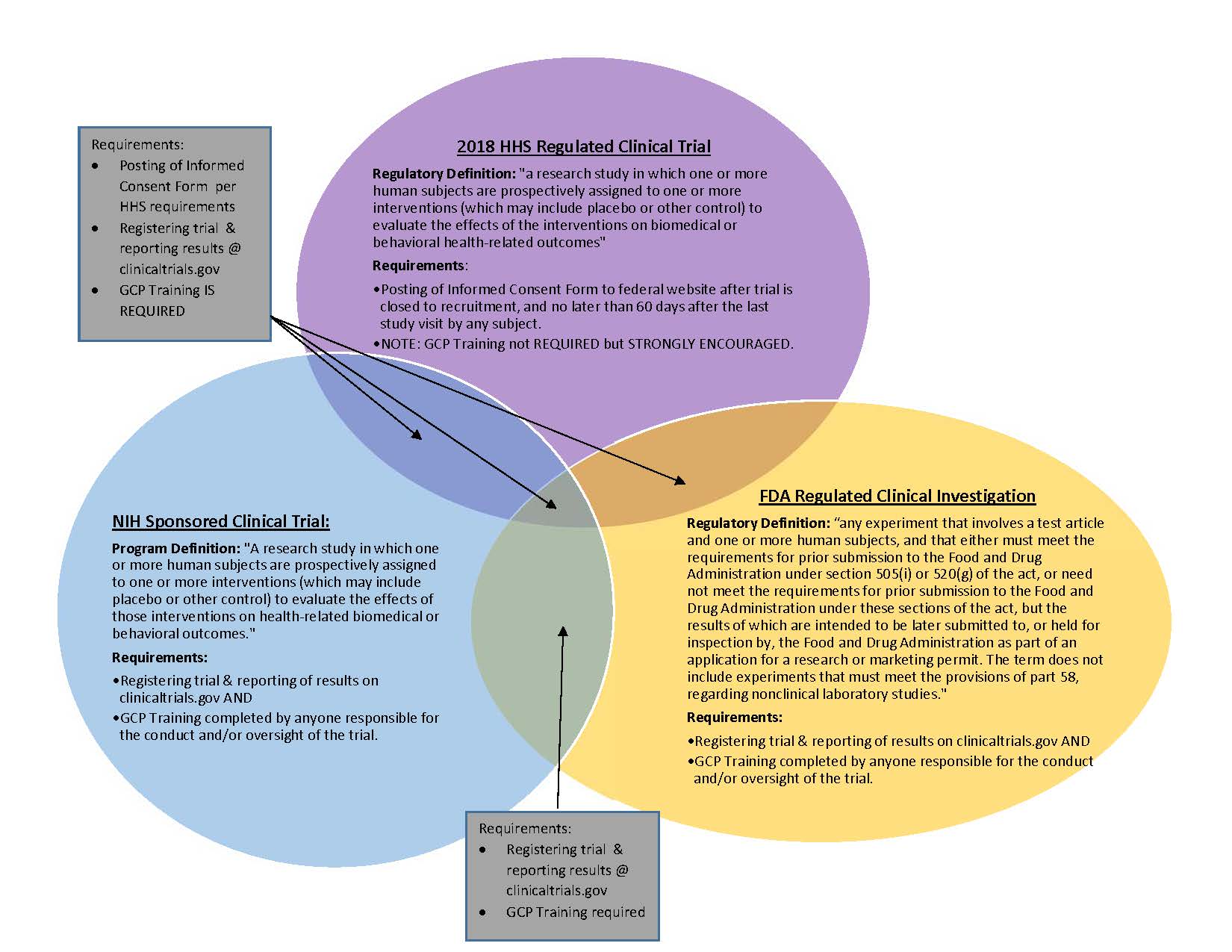
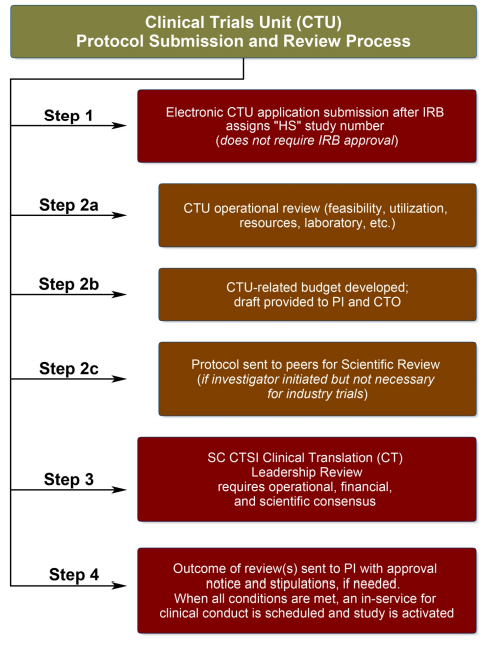
ADVANCED CLINICAL TRIALS THE CLINICAL TRIAL PROCESS: IMPENDING CHANGES IN THE REGULATORY FRAMEWORK - ADVANCED CLINICAL TRIALS

CT01: How to Gain and Maintain Approval for Clinical Research Under the EU Clinical Trials Directive | Zenosis – Learning for Life

Improving the Quality Conduct and Efficiency of Clinical Trials with Training: Recommendations for Qualification of Investigators and Delegates | Semantic Scholar
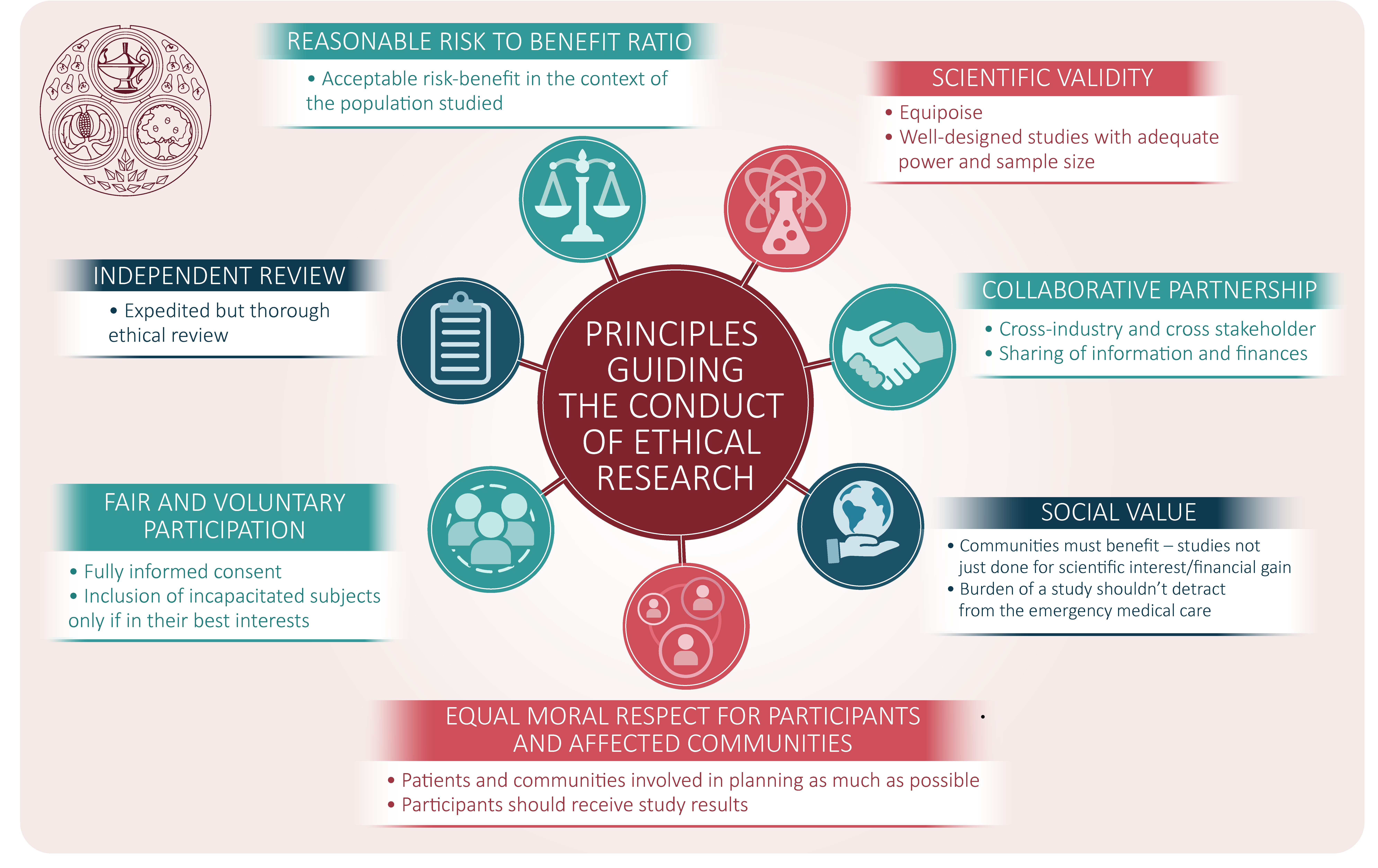
The ethics of conducting clinical trials in the search for treatments and vaccines against COVID-19 - FPM

Improving and sustaining the site investigator community: Recommendations from the Clinical Trials Transformation Initiative - ScienceDirect