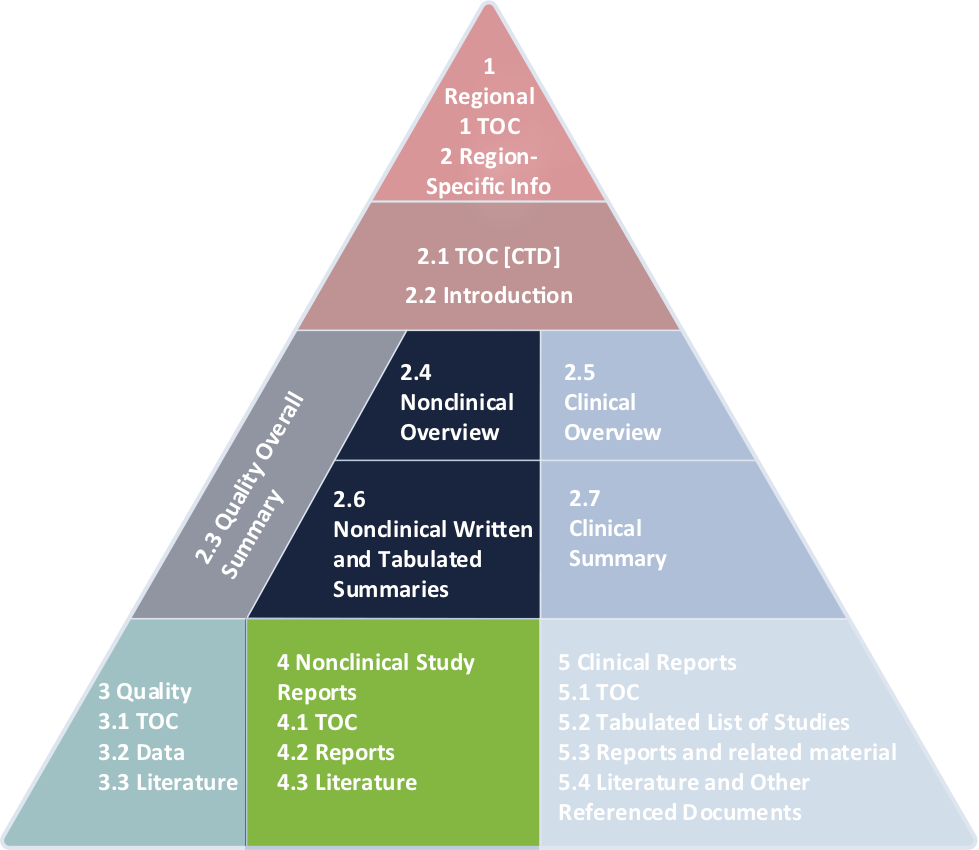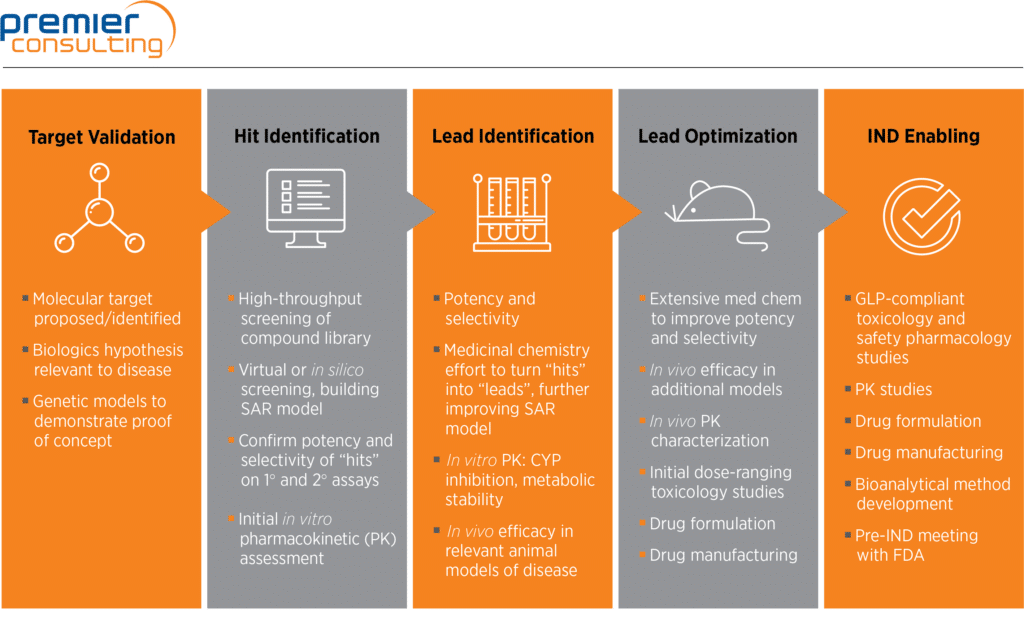
Nonclinical Development Basics: The Right Nonclinical Strategy And Study Designs | Premier Consulting

Statistical controversies in clinical research: limitations of open-label studies assessing antiangiogenic therapies with regard to evaluation of vascular adverse drug events—a meta-analysis - Annals of Oncology